 |
|||||||||||||||||||||||||
 | |||||||||||||||||||||||||
List of CE marking conformity modules applicable for Medical Device (MD) and In Vitro Diagnostic (IVD) Medical DeviceFAQ/Q&A: Questions and Answers about CE Marking of Medical Devices
List of CE marking conformity modules applicable for Medical Devices (MD) and In Vitro Diagnostic (IVD) Medical DevicesModule A Internal Control of Production. Covers internal design and production control. Does normally not require a Notified Body to take action. Within medical devices directives, Notified Body action is required for sterile devices and devices with measurement function (compare MDD Annex VII) or in vitro diagnostic medical devices for self-testing (compare Annex III (6) IVDD). Module B EC type-examination. Covers the design phase, and must be followed up by a module providing for assessment in the production phase. Within the medical devices directives Modules C and G are not applicable. Module D Production quality assurance. Covers the production phase and is an approval of the quality system for production, final product inspection and testing set up by the manufacturer. Within IVDD also the verification of manufactured products is part of Module D. Module E Product quality assurance. Covers the production phase and is an approval of the quality system for final product inspection and testing set up by the manufacturer. Module F Product verification. Covers the production phase; verification by examination and testing of every product and also statistical verification are possible. Module H Full quality assurance. Covers the design and production phases. Within the medical device directives Module H consists of EC design examination and an approval of the quality system for design, manufacture, final product inspection and testing set up by the manufacturer. Within IVDD also the verification of manufactured products is part of Module H. 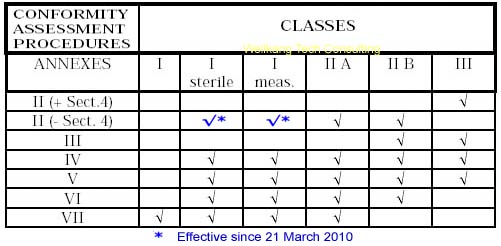 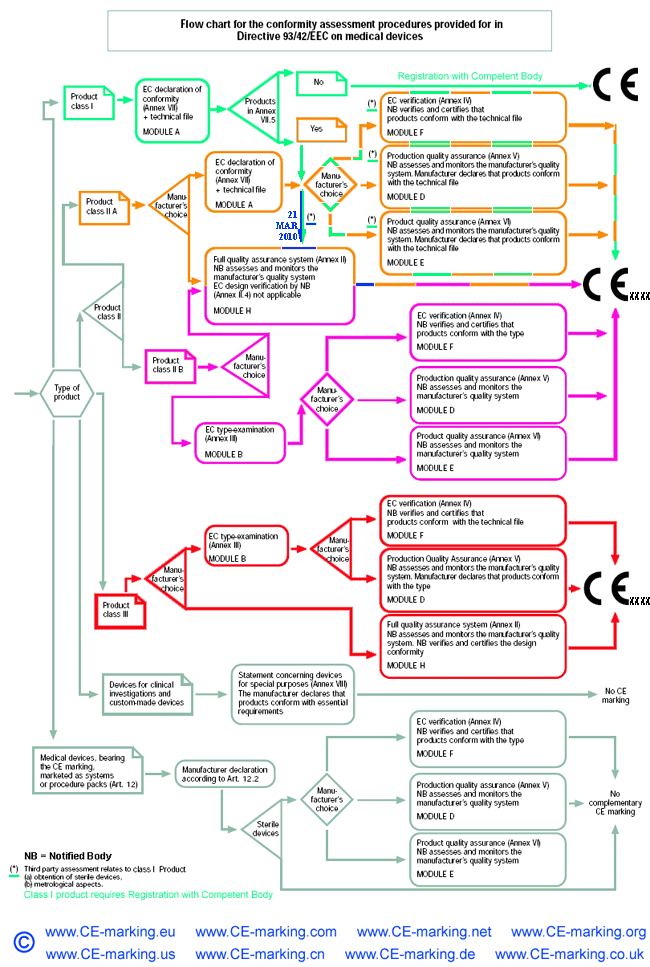 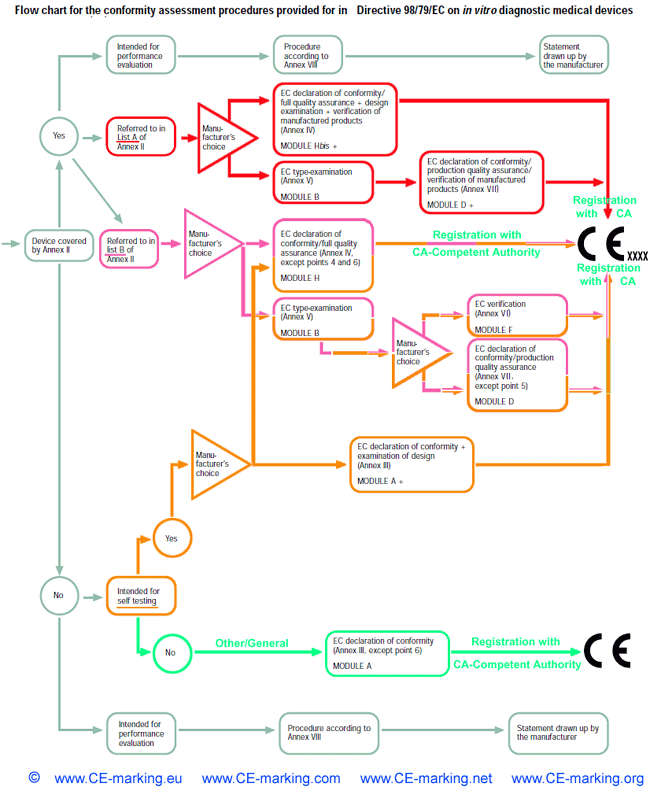  About CE Marking:
|
|||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||